- Choose professional Small Sunflower Seed Oil Refinery Line
- Professionally Designed Mini Oil Mill for Home Use
- Is Small Oil Production Line Worth for Investment?
- How to Build Small Edible Oil Production Line?
- Small Oil Mill Plant for Daily Requirement
- Small Edible Oil Refinery Plant Cost Can Be Reduced Relatively
- How to Extract Palm Oil from Palm Fruit-Oil Mill Plant
- start oil extraction factory
Oil Modification Plant
Some vegetable oils have only limited applications in food products when they are used in their native form. They are therefore often chemically or physically modified in order to change their properties. The most frequently used processes are fractionation and hydrogenation. It seems that, of these technologies, oil fractionation is definitely the ideal choice, thanks to its low operating costs, zero oil loss and reversibility. KMEC supplies a state of the art oil modification plant that is known for its high performance and durability.
Today, however, quite a number of questions have arisen with respect to the effect of chemical modification on the nutritional quality of oils. New technologies have been developed and existing processes have been improved in order to respond to new quality standards. A more integrated and combined use of the different modification technologies has therefore become necessary.
Vegetable Oil Hydrogenation
Hydrogenation is an oils and fats modification process. It is mainly used on vegetable oils derived from soybeans, rape, cotton or sunflower to increase their oxidative stability and to improve their melting properties by reducing their degree of unsaturation. As a result, these oils or fats reach a consistency that is ideal for use as margarine or shortening components. Hydrogenation is a reaction involving the use of a catalyst, usually nickel, and is also an exothermic reaction, thus vegetable oil hydrogenation is an important process in oil modification.
Oil Fractionation
In the field of edible oil processing plant, fractionation almost always refers to the mechanical separation of the liquid from the solid, crystallised, as constituents of a given oil or fat. The split between liquid and solid fractions depends on the temperature at which crystallization is carried out.
Flowchart of Oil Fractionation
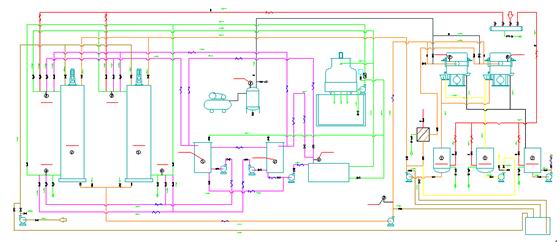
Oil Fractionation Workshop

Fractionation is a process that has been known in industrial form for more than a century. Before then, the liquid (olein) and solid (stearin) fractions of certain oils and fats had been separated by settling, using only the force of gravity to bring about a separation between the heavier solid phase and the lighter liquid phase. Naturally this method of fractionating left the settled solid phase containing large quantities of entrained (trapped) liquid oil; almost certainly more than 75%. In later years a process of this type, using only indirect cooling of the oil but separating liquid from solid by filter or centrifuge, became known as 'dry fractionation'.
The fractionation process can either be used as a stand-alone process (it is normally applied to refined oils) or as part of a more complex process. Thus it can be coupled with hydrogenation, either with hydrogenation being a first stage followed by fractionation, e.g. in the case of hardening of soybean oil to eliminate linolenic acid, followed by fractionation to separate the stearin formed, or with hydrogenation applied to one of the fractions produced in a fractionation process. An example of the latter combination is the hardening of palm kernel stearin produced by fractionation to near- zero iodine value. The fractionation process can also be coupled with interesterification, either in order to randomize a fraction obtained in a fractionation process or to interesterify a blend, one component of which is a fraction produced by fractionation.

 Français
Français Русский
Русский Español
Español
